Why Ice floats on Water?
Why ice floats on water?
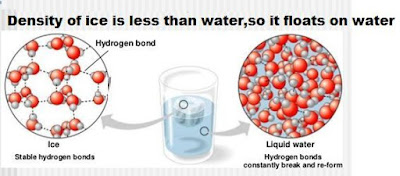
As we know solid objects are more denser than liquid objects but ice floats on water.Its amazing things in the nature.Before we know the science behind it,we have to know the word 'density'.Density is the mass divided by volume.Ice floats because it is less dense then the water.Water has a density of 1.0 gm/cubic cm.The density of ice is 0.931 gm/cubic cm.But, why is ice less dense than water if both are made up of molecules of H2O ?
The reason of ice floats on water is because of hydrogen bond.Hydrogen bond forms between the hydrogen in a water molecule and oxygen in a different water molecule.In water the formed new bonds of molecules try to getting a stable crystal structure.By decreasing temperature of water the molecules apart from each other and increases the inter molecular space resulting the expansion.Therefor the density of ice increases compare to the water.This causes ice float on water.



Well explained.
ReplyDelete